Search results
Search for "halogen bond" in Full Text gives 30 result(s) in Beilstein Journal of Organic Chemistry.
Green and sustainable approaches for the Friedel–Crafts reaction between aldehydes and indoles
- Periklis X. Kolagkis,
- Eirini M. Galathri and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2024, 20, 379–426, doi:10.3762/bjoc.20.36
- electron-withdrawing substituents. The reaction mechanism is based on the activation of the carbonyl group by molecular I2, through the formation of a halogen bond, which lowers the LUMO of the carbonyl moiety, increasing its electrophilicity, and thus allowing the addition of the indole group (Scheme 7
- reaction of aldehydes and ketones with indole [93][94]. Bidentate halogen-bond donors are efficient catalysts, since they can form two halogen bonds with each substrate, instead of just one. Thus, compounds 24, 25 and 26 were screened for their catalytic activity with 26 emerging as the optimum choice
- (Scheme 10). With catalyst 26 prepared, its use was then studied as a halogen-bond donor in the catalytic synthesis of 28 (Scheme 11) [93][94]. Having identified the optimum reaction conditions, the general applicability was studied by reacting various indoles with a range of aldehydes and ketones to

Graphical Abstract

Scheme 1: Examples of BIMs used for their medicinal properties.

Scheme 2: Mechanisms for the synthesis of BIMs using protic or Lewis acids as catalysts.

Scheme 3: Synthesis of bis(indolyl)methanes using DBDMH.

Scheme 4: Competition experiments and synthesis of bis(indolyl)methanes using DBDMH.

Scheme 5: Proposed mechanism for formation of BIM of using DBDMH.

Scheme 6: Synthesis of bis(indolyl)methanes using I2.

Scheme 7: General reaction mechanism upon halogen bonding.

Scheme 8: Synthesis of bis(indolyl)methanes using I2, introduced by Ji.

Scheme 9: Synthesis of bis(indolyl)methanes using Br2 in CH3CN.

Scheme 10: Βidentate halogen-bond donors.

Scheme 11: Synthesis of bis(indolyl)methanes using bidentate halogen-bond donor 26.

Scheme 12: Proposed reaction mechanism.

Scheme 13: Synthesis of bis(indolyl)methanes using iodoalkyne as catalyst.

Scheme 14: Proposed reaction mechanism.

Scheme 15: Optimized reaction conditions used by Ramshini.

Scheme 16: Activation of the carbonyl group by HPA/TPI-Fe3O4.

Scheme 17: Synthesis of BIMs in the presence of nanoAg-Pt/SiO2-doped silicate.

Scheme 18: Mechanism of action proposed by Khalafi-Nezhad et al.

Scheme 19: Activation of the carbonyl group by the Cu–isatin Schiff base complex.

Scheme 20: Optimum reaction conditions published by Jain.

Scheme 21: Organocatalytic protocol utilizing nanoparticles introduced by Bankar.

Scheme 22: Activation of the carbonyl group by the AlCl3·6H2O-SDS-SiO2 complex.

Scheme 23: Optimal reaction conditions for the aforementioned nano-Fe3O4 based catalysts.

Scheme 24: Nanocatalytic protocol proposed by Kaur et al.

Scheme 25: Microwave approach introduced by Yuan.

Scheme 26: Microwave approach introduced by Zahran et al.

Scheme 27: Microwave irradiation protocol introduced by Bindu.

Scheme 28: Silica-supported microwave irradiation protocol.

Scheme 29: Proposed mechanism for formation of BIM by Nongkhlaw.

Scheme 30: Microwave-assisted synthesis of BIMs catalyzed by succinic acid.

Scheme 31: Proposed mechanism of action of MMO-4.

Scheme 32: Catalytic approach introduced by Muhammadpoor-Baltork et al.

Scheme 33: Reaction conditions used by Xiao-Ming.

Scheme 34: Ultrasonic irradiation-based protocol published by Saeednia.

Scheme 35: Pyruvic acid-mediated synthesis of BIMs proposed by Thopate.

Scheme 36: Synthesis of BIMs using [bmim]BF4 or [bmim]PF6 ionic liquids.

Scheme 37: Synthesis of BIMs utilizing In(OTf)3 in octylmethylimidazolium hexafluorophosphate as ionic liquid.

Scheme 38: FeCl3·6H2O-catalyzed synthesis of BIMs with use of ionic liquid.

Scheme 39: Synthesis of BIMs utilizing the [hmim]HSO4/EtOH catalytic system.

Scheme 40: Synthesis of BIMs utilizing acidic ionic liquid immobilized on silica gel (ILIS-SO2Cl).

Scheme 41: The [bmim][MeSO4]-catalyzed reaction of indole with various aldehydes.

Scheme 42: The role of [bmim][MeSO4] in catalyzing the reaction of indole with aldehydes.

Scheme 43: Synthesis of BIMs utilizing FeCl3-based ionic liquid ([BTBAC]Cl-FeCl3) as catalyst.

Scheme 44: Synthesis of BIMs using [Msim]Cl at room temperature.

Scheme 45: [Et3NH][H2PO4]-catalyzed synthesis of bis(indolyl)methanes.

Scheme 46: PILs-catalyzed synthesis of bis(indolyl)methanes.

Scheme 47: FSILs-mediated synthesis of bis(indolyl)methanes.

Scheme 48: Possible “release and catch” catalytic process.

Scheme 49: Synthesis of bis(indolyl)methanes by [DABCO-H][HSO4].

Scheme 50: Synthesis of bis(indolyl)methanes by [(THA)(SO4)].

Scheme 51: Synthesis of BBSI-Cl and BBSI-HSO4.

Scheme 52: Synthesis of BIMs in the presence of BBSI-Cl and BBSI-HSO4.

Scheme 53: Chemoselectivity of the present method.

Scheme 54: Synthesis of BIMs catalyzed by chitosan-supported ionic liquid.

Scheme 55: Proposed mechanism of action of CSIL.

Scheme 56: Optimization of the reaction in DESs.

Scheme 57: Synthesis of BIMs using ChCl/SnCl2 as DES.

Scheme 58: Synthesis of BIMs derivatives in presence of DES.

Scheme 59: BIMs synthesis in choline chloride/urea (CC/U).

Scheme 60: Flow chemistry-based synthesis of BIMs by Ley.

Scheme 61: Flow chemistry-based synthesis of BIMs proposed by Nam et al.

Scheme 62: Amino-catalyzed reaction of indole with propionaldehyde.

Scheme 63: Aminocatalytic synthesis of BIMs.

Scheme 64: Proposed mechanism for the aminocatalytic synthesis of BIMs.

Scheme 65: Enzymatic reaction of indole with aldehydes.

Scheme 66: Proposed mechanism for the synthesis of BIMs catalyzed by TLIM.

Scheme 67: Proposed reaction mechanism by Badsara.

Scheme 68: Mechanism proposed by D’Auria.

Scheme 69: Photoinduced thiourea catalysis.

Scheme 70: Proposed mechanism of photoacid activation.

Scheme 71: Proposed mechanism of action for CF3SO2Na.

Scheme 72: Proposed mechanism for the synthesis of BIMs by Mandawad.

Scheme 73: Proposed mechanism for the (a) acid generation and (b) synthesis of BIMs.

Scheme 74: a) Reaction conditions employed by Khaksar and b) activation of the carbonyl group by HFIP.

Scheme 75: Activation of the carbonyl group by the PPy@CH2Br through the formation of a halogen bond.

Scheme 76: Reaction conditions utilized by Mhaldar et al.

Scheme 77: a) Reaction conditions employed by López and b) activation of the carbonyl group by thiourea.

Scheme 78: Infrared irradiation approach introduced by Luna-Mora and his research group.

Scheme 79: Synthesis of BIMs with the use of the Fe–Zn BMOF.
Exploring the role of halogen bonding in iodonium ylides: insights into unexpected reactivity and reaction control
- Carlee A. Montgomery and
- Graham K. Murphy
Beilstein J. Org. Chem. 2023, 19, 1171–1190, doi:10.3762/bjoc.19.86

- pattern shown in Figure 1, where R is the host atom or functional group to which the halogen is covalently bound, where X is the halogen atom possessing the σ-hole (halogen bond donor), and where Y is the Lewis base (halogen bond acceptor) [31]. σ-Holes arise from anisotropic covalent bonds between the
- influence σ-hole strength [32][48]. Should the halogen possess two covalent bonds, two σ-holes would result with the stronger situated opposite the more electron-attracting group [24]. σ-Holes are also highly directional, with near linear halogen bond-acceptor approach angles that typically fall between 160
- –180° [39][49][50], and with halogen bond lengths that are typically less than or equal to the sum of the atomic Van der Waals radii of the engaged atoms [33]. The strength of a subsequent halogen bond is influenced by the magnitudes of the positive electrostatic potential (VS,max) of the donor and the

Graphical Abstract

Figure 1: Generic representation of halogen bonding.

Figure 2: Quantitative evaluation of σ-holes in monovalent iodine-containing compounds; and, qualitative mole...

Figure 3: Quantitative evaluation of σ-holes in hypervalent iodine-containing molecules; and, qualitative MEP...

Figure 4: Quantitative evaluation of σ-holes in iodonium ylides; and, qualitative MEP map of I-12 from −0.083...

Scheme 1: Outline of possible reaction pathways between iodonium ylides and Lewis basic nucleophiles (top); a...

Scheme 2: Metal-free cyclopropanations of iodonium ylides, either as intermolecular (a) or intramolecular pro...

Figure 5: Zwitterionic mechanism for intramolecular cyclopropanation of iodonium ylides (left); and, stepwise...

Scheme 3: Metal-free intramolecular cyclopropanation of iodonium ylides.

Figure 6: Concerted cycloaddition pathway for the metal-free, intramolecular cyclopropanation of iodonium yli...

Scheme 4: Reaction of ylide 6 with diphenylketene to form lactone 24 and 25.

Figure 7: Nucleophilic (top) and electrophilic (bottom) addition pathways proposed by Koser and Hadjiarapoglo...

Scheme 5: Indoline synthesis from acyclic iodonium ylide 31 and tertiary amines.

Scheme 6: N-Heterocycle synthesis from acyclic iodonium ylide 31 and secondary amines.

Figure 8: Proposed mechanism for the formation of 33a from iodonium ylides and amines, involving an initial h...

Scheme 7: Indoline synthesis from acyclic iodonium ylides 39 and tertiary amines under blue light photocataly...

Scheme 8: Metal-free cycloproponation of iodonium ylides under blue LED irradiation. aUsing trans-β-methylsty...

Figure 9: Proposed mechanism of the cyclopropanation between iodonium ylides and alkenes under blue LED irrad...

Scheme 9: Formal C–H alkylation of iodonium ylides by nucleophilic heterocycles under blue LED irradiation.

Figure 10: Proposed mechanism of the formal C–H insertion of pyrrole under blue LED irradiation.

Scheme 10: X–H insertions between iodonium ylides and carboxylic acids, phenols and thiophenols.

Figure 11: Mechanistic proposal for the X–H insertion reactions of iodonium ylides.

Scheme 11: Radiofluorination of biphenyl using iodonium ylides 54a–e derived from various β-dicarbonyl auxilia...

Scheme 12: Radiofluorination of arenes using spirocycle-derived iodonium ylides 56.

Scheme 13: Radiofluorination of arenes using SPIAd-derived iodonium ylides 58.

Figure 12: Calculated reaction coordinate for the radiofluorination of iodonium ylide 60.

Scheme 14: Radiofluorination of iodonium ylides possessing various ortho- and para-substituents on the iodoare...

Figure 13: Difference in Gibbs activation energy for ortho- or para-anisyl derived iodonium ylides 63a and 63b....

Figure 14: Proposed equilibration of intermediates to transit between 64a (the initial adduct formed between 6...

Scheme 15: Comparison of 31 and ortho-methoxy iodonium ylide 39 in rhodium-catalyzed cyclopropanation and cycl...

Figure 15: X-ray crystal structure of dimeric 39 [6], (CCDC# 893474) [143,144].

Scheme 16: Enaminone synthesis using diazonium and iodonium ylides.

Figure 16: Transition state calculations for enaminone synthesis from iodonium ylides and thioamides.

Scheme 17: The reaction between ylides 73a–f and N-methylpyrrole under 365 nm UV irradiation.

Figure 17: Crystal structures of 76c (top) and 76e (bottom) [101], (CCDC# 2104180 & 2104181) [143,144].
Photoredox catalysis harvesting multiple photon or electrochemical energies
- Mattia Lepori,
- Simon Schmid and
- Joshua P. Barham
Beilstein J. Org. Chem. 2023, 19, 1055–1145, doi:10.3762/bjoc.19.81

Graphical Abstract

Figure 1: Oxidative and reductive activations of organic compounds harvesting photoredox catalysis.

Figure 2: General catalytic cycles of radical ion conPET (left) and radical ion e-PRC (right).

Figure 3: “Beginner’s guide”: comparison between advantages, capacities, and prospectives of conPET and PEC.

Figure 4: A) conPET reductive dehalogenation of aryl halides with PDI. B) Reductive C–H arylation with pyrrol...

Figure 5: A) Chromoselective mono- and disubstitution or polybrominated pyrimidines with pyrroles. B) Sequent...

Figure 6: A) Synthesis of pyrrolo[1,2-a]quinolines. B) Synthesis of ullazines.

Figure 7: A) Reductive phosphorylation of aryl halides via conPET. B) Selected examples from the substrate sc...

Figure 8: A) Reductive dehalogenation of aryl halides via conPET and selected examples from the substrate sco...

Figure 9: A) Reductive C–H arylation of aryl halides via conPET (top) and selected examples from the substrat...

Figure 10: A) Reductive hydrodehalogenation of aryl halides with Mes-Acr-BF4. B) Selected examples from the su...

Figure 11: A) Reductive hydrodechlorination of aryl chlorides with 4-DPAIPN. B) Proposed formation of CO2•−. C...

Figure 12: A) Reductive conPET borylation with 3CzEPAIPN (top) and selected examples from the substrate scope ...

Figure 13: Scale-up of conPET phosphorylation with 3CzEPAIPN.

Figure 14: A) Borylation of 1d. B) Characteristics and structure of PC1 with green and red parts showing the l...

Figure 15: A) Reductive C–H arylation scope with polysulfide conPET (top) and selected examples from the subst...

Figure 16: Scale-up of A) C–H arylation and B) dehaloborylation with polysulfide photocatalysis in continuous-...

Figure 17: A) Formation of [Ir1]0 and [Ir2]0 upon PET between [Ir1]+ and Et3N. B) Mechanism of multi-photon ta...

Figure 18: A) Reductive hydrodehalogenation of aryl halides via multi-photon tandem photocatalysis. B) Selecte...

Figure 19: A) Carbonylative amidation of aryl halides in continuous flow. B) Selected examples from the substr...

Figure 20: A) General scheme for reductive (RQ) and oxidative quenching (OQ) protocols using [FeIII(btz)3](PF6)...

Figure 21: A) Carbonylative amidation of alkyl iodides with [IrIII(ppy)2(dtbbpy)]PF6. B) Selected examples fro...

Figure 22: A) Carboxylative C–N bond cleavage in cyclic amines. B) Selected examples from the substrate scope....

Figure 23: A) Formal reduction of alkenes to alkanes via transfer hydrogenation. B) Selected examples from the...

Figure 24: A) Birch-type reduction of benzenes with PMP-BPI. B) Selected examples from the substrate scope (sc...

Figure 25: Proposed mechanism of the OH− mediated conPET Birch-type reduction of benzene via generation of sol...

Figure 26: Reductive detosylation of N-tosylated amides with Mes-Acr-BF4. B) Selected examples from the substr...

Figure 27: A) Reductive detosylation of N-tosyl amides by dual PRC. B) Selected examples from the substrate sc...

Figure 28: A) Mechanism of the dual PRC based on PET between [Cu(dap)2]+ and DCA. B) Mechanism of the dual PRC...

Figure 29: A) N–O bond cleavage in Weinreb amides with anthracene. B) N–O bond cleavage in Weinreb amides rely...

Figure 30: A) Pentafluorosulfanylation and fluoride elimination. B) Mechanism of the pentafluorosulfanylation ...

Figure 31: A) α-Alkoxypentafluorosulfanylation (top) and selected examples from the substrate scope (bottom). ...

Figure 32: A) Oxidative amination of arenes with azoles catalyzed by N-Ph PTZ. B) Selected examples from the s...

Figure 33: A) C(sp3)–H bond activation by HAT via chloride oxidation by *N-Ph PTZ•+. B) Proposed mechanism for...

Figure 34: A) Recycling e-PRC C–H azolation of electron-rich arenes with pyrazoles using Mes-Acr+ as a photoca...

Figure 35: A) Radical ion e-PRC direct oxidation of unactivated arenes using TAC+ as an electro-activated phot...

Figure 36: A) Radical ion e-PRC direct oxidation of unactivated arenes using TPA as an electro-activated photo...

Figure 37: Proposed mechanism (top) and mode of preassembly (bottom).

Figure 38: A) Possible preassemblies of reactive (left) vs unreactive (right) arenes. B) Calculated spin densi...

Figure 39: A) Recycling e-PRC C(sp2 )–H acetoxylation of arenes using DDQ as a photocatalyst. B) Proposed cata...

Figure 40: Gram scale hydroxylation of benzene in a recirculated flow setup.

Figure 41: A) Radical ion e-PRC vicinal diamination of alkylarenes using TAC+ as an electro-activated photocat...

Figure 42: A) Sequential oxygenation of multiple adjacent C–H bonds under radical ion e-PRC using TAC+ as an e...

Figure 43: A) Enantioselective recycling e-PRC cyanation of benzylic C–H bonds using ADQS as photocatalyst. B)...

Figure 44: Proposed tandem mechanism by Xu and co-workers.

Figure 45: A) Enantioselective recycling e-PRC decarboxylative cyanation using Cu(acac)2, Ce(OTf)3 and a box l...

Figure 46: A) Enantioselective recycling e-PRC benzylic cyanation using Cu(MeCN)4BF4, box ligand and anthraqui...

Figure 47: A) Radical ion e-PRC acetoxyhydroxylation of aryl olefins using TAC+ as an electro-activated photoc...

Figure 48: Selected examples from the substrate scope.

Figure 49: Photoelectrochemical acetoxyhydroxylation in a recirculated flow setup.

Figure 50: A) Radical ion e-PRC aminooxygenation of aryl olefins using TAC+ as an electro-activated photocatal...

Figure 51: A) Recycling e-PRC C–H alkylation of heteroarenes with organic trifluoroborates using Mes-Acr+ as p...

Figure 52: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using CeCl3·7H2O as catalyst. B) ...

Figure 53: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using Fe(NH4)2(SO4)2·6H2O as cata...

Figure 54: A) Recycling e-PRC C–H alkylation of heteroarenes with alkyl oxalates and 4CzIPN as photocatalyst. ...

Figure 55: A) Recycling e-PRC decarboxylative C–H carbamoylation of heteroarenes using 4CzIPN as photocatalyst...

Figure 56: A) Photoelectrochemical HAT-mediated hydrocarbon activation via the chlorine radical. B) Proposed m...

Figure 57: A) Selected examples from the substrate scope. B) Gram and decagram scale semi-continuous flow PEC ...

Figure 58: A) Photoelectrochemical HAT-mediated dehydrogenative coupling of benzothiazoles with aliphatic C–H ...

Figure 59: A) Photoelectrochemical HAT activation of ethers using electro-activated TAC+ as photocatalyst. B) ...

Figure 60: Selected examples from the substrate scope.

Figure 61: A) Photoelectrochemical HAT-mediated synthesis of alkylated benzimidazo-fused isoquinolinones using...

Figure 62: A) Decoupled photoelectrochemical cerium-catalyzed oxydichlorination of alkynes using CeCl3 as cata...

Figure 63: Proposed decoupled photoelectrochemical mechanism.

Figure 64: A) Decoupled photoelectrochemical ring-opening bromination of tertiary cycloalkanols using MgBr2 as...

Figure 65: A) Recycling e-PRC ring-opening functionalization of cycloalkanols using CeCl3 as catalyst. B) Prop...

Figure 66: Selected examples from the substrate scope of the PEC ring-opening functionalization.

Figure 67: A) Radical ion e-PRC reduction of chloro- and bromoarenes using DCA as catalyst and various accepto...

Figure 68: A) Screening of different phthalimide derivatives as catalyst for the e-PRC reduction of aryl halid...

Figure 69: Screening of different organic catalysts for the e-PRC reduction of trialkylanilium salts.

Figure 70: A) e-PRC reduction of phosphonated phenols and anilinium salts. B) Selected examples from the subst...

Figure 71: A) ConPET and e-PRC reduction of 4-bromobenzonitrile using a naphthalene diimide (NDI) precatalyst ...

Figure 72: A) Radical ion e-PRC reduction of phosphinated aliphatic alcohols with n-BuO-NpMI as catalyst. B) C...

Figure 73: Selected examples from the substrate scope.

Figure 74: A) Recycling e-PRC reductive dimerization of benzylic chlorides using a [Cu2] catalyst. B) Proposed...

Figure 75: A) Decoupled photoelectrochemical C–H alkylation of heteroarenes through deamination of Katritzky s...

Figure 76: Proposed mechanism by Chen and co-workers.
Synthesis and reactivity of azole-based iodazinium salts
- Thomas J. Kuczmera,
- Annalena Dietz,
- Andreas Boelke and
- Boris J. Nachtsheim
Beilstein J. Org. Chem. 2023, 19, 317–324, doi:10.3762/bjoc.19.27
- aryl donors [11][12][13][14][15][16]. Their cyclic derivatives have a proven utility as precursors for the synthesis of hetero- and carbocycles [17][18][19][20][21], and their pronounced σ-holes [22] render them efficient halogen-bond donors (XB donors in XB catalysis) [23]. Despite their great

Graphical Abstract

Figure 1: Nitrogen-containing iodolium and iodonium salts.

Figure 2: Synthesis of a set of azoiodazinium salts 5. Method A: Iodoarene 4 (200 µmol) and mCPBA (1.1 equiv)...

Figure 3: Single crystal structures (ORTEP drawing with 50% probability) of the pyrazole-coordinated salt 5bb...

Scheme 1: Derivatizations of the iodonium salt 5aa. a) Ac2O, CuSO4·5H2O, NaOAc, AcOH, 120 °C, 5 h; b) S8/Se/T...

Scheme 2: Post-functionalization of mono- and dicationic iodonium salts under preservation of the hypervalent...
BINOL as a chiral element in mechanically interlocked molecules
- Matthias Krajnc and
- Jochen Niemeyer
Beilstein J. Org. Chem. 2022, 18, 508–523, doi:10.3762/bjoc.18.53

- -workers, with a strong focus on using rotaxanes with halogen-bond (XB) donors that act as binding sites for anionic guest molecules [23]. In 2017, Beer and co-workers reported the synthesis of the BINOL-containing chiral [2]rotaxanes 64 and their application for enantioselective anion recognition [63

Graphical Abstract

Figure 1: Molecular structures of (R)-BINOL (left) and (S)-BINOL (right).

Figure 2: Synthesis of Sauvage´s [2]catenanes (S,S)-5 and (S,S)-6 containing two BINOL units by the passive m...

Figure 3: Synthesis of Saito´s [2]rotaxane (R)-10 from a BINOL-based macrocycle by the active metal template ...

Figure 4: Synthesis of Stoddart´s [2]rotaxane (rac)-14 by an ammonium crown ether template.

Figure 5: Synthesis of Stoddart´s BINOL-containing [2]catenanes 18/20/22/24 by π–π recognition.

Figure 6: Synthesis of Takata´s rotaxanes featuring chiral centers on the axle: a) rotaxane (R,R,R/S)-27 obta...

Figure 7: Takata´s chiral polyacetylenes 32/33 featuring BINOL-based [2]rotaxane side chains.

Figure 8: Synthesis of Takata´s chiral thiazolium [2]rotaxanes (R)-35a/b and (R)-38.

Figure 9: Results for the asymmetric benzoin condensation of benzaldehyde (39) with catalysts (R)-35a/b and (R...

Figure 10: Synthesis of Takata´s pyridine-based [2]rotaxane (R)-42.

Figure 11: The asymmetric desymmetrization reaction of meso-1,2-diols with rotaxane (R)-42.

Figure 12: Synthesis of Niemeyer´s axially chiral [2]catenane (S,S)-47.

Figure 13: Results for the enantioselective transfer hydrogenation of 2-phenylquinoline with catalysts (S,S)-47...

Figure 14: Synthesis of Niemeyer´s chiral [2]rotaxanes (S)-56/57.

Figure 15: Results for the enantioselective Michael addition with different rotaxane catalysts (S)-56a/56b/57a/...

Figure 16: Synthesis of Beer´s [2]rotaxanes 64a/b for anion recognition.

Figure 17: Association constants of different anions (used as the Bu4N+ salts) to the [2]rotaxanes (S)-64a/b a...

Figure 18: Synthesis of Beer´s [3]rotaxane (S)-68.

Figure 19: Association constants of different anions (used as the Bu4N+-salts) to the [2]rotaxane (S)-68 and a...
Mechanistic studies of the solvolysis of alkanesulfonyl and arenesulfonyl halides
- Malcolm J. D’Souza and
- Dennis N. Kevill
Beilstein J. Org. Chem. 2022, 18, 120–132, doi:10.3762/bjoc.18.13
- and Scott showed [9] that in 50% acetone/50% water at 0.5 °C, a hydroxide ion was about 106 times more reactive than a water molecule towards benzenesulfonyl chloride. Also, for the same solvent, at 25.0 °C, they found [10] that the fluoride, with a considerably stronger carbon–halogen bond, reacted

Graphical Abstract

Scheme 1: Organic reactions where the breaking of a C–X bond involves the formation of a high energy ion-pair...

Scheme 2: The chemical structures for the 1-adamantyl substrate, 2-adamantyl substrate, and the S-methyldiben...

Figure 1: The SN2 reaction plot of log (k/ko) vs (1.26 NT + 0.65 YCl) for the solvolyses of benzesulfonyl chl...

Figure 2: The SN2 reaction plot of log (k/ko) vs (1.35 NT + 0.70 YCl) for the solvolyses of 2-thiophenesulfon...
Halides as versatile anions in asymmetric anion-binding organocatalysis
- Lukas Schifferer,
- Martin Stinglhamer,
- Kirandeep Kaur and
- Olga García Macheño
Beilstein J. Org. Chem. 2021, 17, 2270–2286, doi:10.3762/bjoc.17.145
- -trifluorophenyl-type catalysts [45]. Additionally, the first asymmetric systems involving purely halogen bond donor catalysis have recently been developed by the groups of Huber [46] and García Mancheño [47]. Moreover, though chloride as halide counter-anion still being particularly prominent, the application of

Graphical Abstract

Figure 1: a) Binding interactions in the chloride channel of E. coli. and b) examples of chloride, cyanide, n...

Figure 2: a) H-bond vs anion-binding catalysis and b) activation modes in anion-binding catalysis.

Scheme 1: First proposed anion-binding mechanism in the thiourea-catalyzed acetalization of benzaldehyde.

Scheme 2: a) Thiourea-catalyzed enantioselective acyl-Pictet–Spengler reaction of tryptamine-derived imines 4...

Scheme 3: Proposed mechanism of the thiourea-catalyzed enantioselective Pictet–Spengler reaction of hydroxyla...

Scheme 4: a) Thiourea-catalyzed intramolecular Pictet–Spengler-type cyclization of hydroxylactam-derived N-ac...

Scheme 5: Enantioselective Reissert-type reactions of a) (iso)quinolines with silyl ketene acetals, and b) vi...

Figure 3: Role of the counter-anion: a) Anion acting as a spectator and b) anion participating directly as th...

Scheme 6: Enantioselective selenocyclization catalyzed by squaramide 28.

Scheme 7: Desymmetrization of meso-aziridines catalyzed by bifunctional thiourea catalyst 31.

Scheme 8: Anion-binding-catalyzed desymmetrization of a) meso-aziridines catalyzed by chiral triazolium catal...

Scheme 9: Bis-urea-catalyzed enantioselective fluorination of a) β-bromosulfides and b) β-haloamines by Gouve...

Scheme 10: a) Bifunctional thiourea anion-binding – basic/nucleophilic catalysts. Selected applications in b) ...

Scheme 11: Thiourea-catalyzed enantioselective polycyclization reaction of hydroxylactams 51 through cation–π ...

Scheme 12: Enantioselective aza-Sakurai cyclization of hydroxylactams 56 implicating additional cation–π and L...

Scheme 13: Enantioselective tail-to-head cyclization of neryl chloride derivatives.

Scheme 14: Cation–π interactions in anion binding-catalyzed asymmetric addition reactions: a) addition of indo...

Scheme 15: Bisthiourea catalyzed oxa-Pictet–Spengler reaction of indole-based alcohols and aromatic aldehydes ...

Scheme 16: Anion-binding catalyst development in the enantioselective addition of silyl ketene acetals to 1-ch...

Scheme 17: a) Macrocyclic bis-thiourea catalyst in a diastereoselective glycosylation reaction. b) Competing SN...

Scheme 18: a) Folding mechanism of oligotriazoles upon anion recognition. b) Representative tetratriazole 82 c...

Scheme 19: Switchable chiral tetratriazole catalyst 86 in the enantioselective addition of silyl ketene acetal...
Methodologies for the synthesis of quaternary carbon centers via hydroalkylation of unactivated olefins: twenty years of advances
- Thiago S. Silva and
- Fernando Coelho
Beilstein J. Org. Chem. 2021, 17, 1565–1590, doi:10.3762/bjoc.17.112
- hydroalkylation product 92e’, indicating a possible double bond isomerization under these reaction conditions. The postulated mechanism starts with the generation of an ethyl radical from BEt3. This radical then acts as a radical initiator to promote the homolytic cleavage of the carbon–halogen bond of the α-EWG

Graphical Abstract

Figure 1: Some examples of natural products and drugs containing quaternary carbon centers.

Scheme 1: Simplified mechanism for olefin hydrofunctionalization using an electrophilic transition metal as a...

Scheme 2: Selected examples of quaternary carbon centers formed by the intramolecular hydroalkylation of β-di...

Scheme 3: Control experiments and the proposed mechanism for the Pd(II)-catalyzed intermolecular hydroalkylat...

Scheme 4: Intermolecular olefin hydroalkylation of less reactive ketones under Pd(II) catalysis using HCl as ...

Scheme 5: A) Selected examples of Pd(II)-mediated quaternary carbon center synthesis by intermolecular hydroa...

Scheme 6: Selected examples of quaternary carbon center synthesis by gold(III) catalysis. This is the first r...

Scheme 7: Selected examples of inter- (A) and intramolecular (B) olefin hydroalkylations promoted by a silver...

Scheme 8: A) Intermolecular hydroalkylation of N-alkenyl β-ketoamides under Au(I) catalysis in the synthesis ...

Scheme 9: Asymmetric pyrrolidine synthesis through intramolecular hydroalkylation of α-substituted N-alkenyl ...

Scheme 10: Proposed mechanism for the chiral gold(I) complex promotion of the intermolecular olefin hydroalkyl...

Scheme 11: Selected examples of carbon quaternary center synthesis by gold and evidence of catalytic system pa...

Scheme 12: Synthesis of a spiro compound via an aza-Michael addition/olefin hydroalkylation cascade promoted b...

Scheme 13: A selected example of quaternary carbon center synthesis using an Fe(III) salt as a catalyst for th...

Scheme 14: Intermolecular hydroalkylation catalyzed by a cationic iridium complex (Fuji (2019) [47]).

Scheme 15: Generic example of an olefin hydrofunctionalization via MHAT (Shenvi (2016) [51]).

Scheme 16: The first examples of olefin hydrofunctionalization run under neutral conditions (Mukaiyama (1989) [56]...

Scheme 17: A) Aryl olefin dimerization catalyzed by vitamin B12 and triggered by HAT. B) Control experiment to...

Scheme 18: Generic example of MHAT diolefin cycloisomerization and possible competitive pathways. Shenvi (2014...

Scheme 19: Selected examples of the MHAT-promoted cycloisomerization reaction of unactivated olefins leading t...

Scheme 20: Regioselective carbocyclizations promoted by an MHAT process (Norton (2008) [76]).

Scheme 21: Selected examples of quaternary carbon centers synthetized via intra- (A) and intermolecular (B) MH...

Scheme 22: A) Proposed mechanism for the Fe(III)/PhSiH3-promoted radical conjugate addition between olefins an...

Scheme 23: Examples of cascade reactions triggered by HAT for the construction of trans-decalin backbone uniti...

Scheme 24: A) Selected examples of the MHAT-promoted radical conjugate addition between olefins and p-quinone ...

Scheme 25: A) MHAT triggered radical conjugate addition/E1cB/lactonization (in some cases) cascade between ole...

Scheme 26: A) Spirocyclization promoted by Fe(III) hydroalkylation of unactivated olefins. B) Simplified mecha...

Scheme 27: A) Selected examples of the construction of a carbon quaternary center by the MHAT-triggered radica...

Scheme 28: Hydromethylation of unactivated olefins under iron-mediated MHAT (Baran (2015) [95]).

Scheme 29: The hydroalkylation of unactivated olefins via iron-mediated reductive coupling with hydrazones (Br...

Scheme 30: Selected examples of the Co(II)-catalyzed bicyclization of dialkenylarenes through the olefin hydro...

Scheme 31: Proposed mechanism for the bicyclization of dialkenylarenes triggered by a MHAT process (Vanderwal ...

Scheme 32: Enantioconvergent cross-coupling between olefins and tertiary halides (Fu (2018) [108]).

Scheme 33: Proposed mechanism for the Ni-catalyzed cross-coupling reaction between olefins and tertiary halide...

Scheme 34: Proposed catalytic cycles for a MHAT/Ni cross-coupling reaction between olefins and halides (Shenvi...

Scheme 35: Selected examples of the hydroalkylation of olefins by a dual catalytic Mn/Ni system (Shenvi (2019) ...

Scheme 36: A) Selected examples of quaternary carbon center synthesis by reductive atom transfer; TBC: 4-tert-...

Scheme 37: A) Selected examples of quaternary carbon centers synthetized by radical addition to unactivated ol...

Scheme 38: A) Selected examples of organophotocatalysis-mediated radical polyene cyclization via a PET process...

Scheme 39: A) Sc(OTf)3-mediated carbocyclization approach for the synthesis of vicinal quaternary carbon cente...

Scheme 40: Scope of the Lewis acid-catalyzed methallylation of electron-rich styrenes. Method A: B(C6F5)3 (5.0...

Scheme 41: The proposed mechanism for styrene methallylation (Oestreich (2019) [123]).
N-tert-Butanesulfinyl imines in the asymmetric synthesis of nitrogen-containing heterocycles
- Joseane A. Mendes,
- Paulo R. R. Costa,
- Miguel Yus,
- Francisco Foubelo and
- Camilla D. Buarque
Beilstein J. Org. Chem. 2021, 17, 1096–1140, doi:10.3762/bjoc.17.86


Graphical Abstract

Scheme 1: General strategy for the enantioselective synthesis of N-containing heterocycles from N-tert-butane...

Scheme 2: Methodologies for condensation of aldehydes and ketones with tert-butanesulfinamides (1).

Scheme 3: Transition models for cis-aziridines and trans-aziridines.

Scheme 4: Mechanism for the reduction of N-tert-butanesulfinyl imines.

Scheme 5: Transition models for the addition of organomagnesium and organolithium compounds to N-tert-butanes...

Scheme 6: Synthesis of 2,2-dibromoaziridines 15 from aldimines 14 and bromoform, and proposed non-chelation-c...

Scheme 7: Diastereoselective synthesis of aziridines from tert-butanesulfinyl imines.

Scheme 8: Synthesis of vinylaziridines 22 from aldimines 14 and 1,3-dibromopropene 23, and proposed chelation...

Scheme 9: Synthesis of vinylaziridines 27 from aldimines 14 and α-bromoesters 26, and proposed transition sta...

Scheme 10: Synthesis of 2-chloroaziridines 28 from aldimines 14 and dichloromethane, and proposed transition s...

Scheme 11: Synthesis of cis-vinylaziridines 30 and 31 from aldimines 14 and bromomethylbutenolide 29.

Scheme 12: Synthesis of 2-chloro-2-aroylaziridines 36 and 32 from aldimines 14, arylnitriles 34, and silyldich...

Scheme 13: Synthesis of trifluoromethylaziridines 39 and proposed transition state of the aziridination.

Scheme 14: Synthesis of aziridines 42 and proposed state transition.

Scheme 15: Synthesis of 1-substituted 2-azaspiro[3.3]heptanes, 1-phenyl-2-azaspiro[3.4]octane and 1-phenyl-2-a...

Scheme 16: Synthesis of 1-substituted 2,6-diazaspiro[3.3]heptanes 48 from chiral imines 14 and 1-Boc-azetidine...

Scheme 17: Synthesis of β-lactams 52 from chiral imines 14 and dimethyl malonate (49).

Scheme 18: Synthesis of spiro-β-lactam 57 from chiral (RS)-N-tert-butanesulfinyl isatin ketimine 53 and ethyl ...

Scheme 19: Synthesis of β-lactam 60, a precursor of (−)-batzelladine D (61) and (−)-13-epi-batzelladine D (62)...

Scheme 20: Rhodium-catalyzed asymmetric synthesis of 3-substituted pyrrolidines 66 from chiral imine (RS)-63 a...

Scheme 21: Asymmetric synthesis of 1,3-disubstituted isoindolines 69 and 70 from chiral imine 67.

Scheme 22: Asymmetric synthesis of cis-2,5-disubstituted pyrrolidines 73 from chiral imine (RS)-71.

Scheme 23: Asymmetric synthesis of 3-hydroxy-5-substituted pyrrolidin-2-ones 77 from chiral imine (RS)-74.

Scheme 24: Asymmetric synthesis of 4-hydroxy-5-substituted pyrrolidin-2-ones 80 from chiral imines 79.

Scheme 25: Asymmetric synthesis of 3-pyrrolines 82 from chiral imines 14 and ethyl 4-bromocrotonate (81).

Scheme 26: Asymmetric synthesis of γ-amino esters 84, and tetramic acid derivative 86 from chiral imines (RS)-...

Scheme 27: Asymmetric synthesis of α-methylene-γ-butyrolactams 90 from chiral imines (Z,SS)-87 and ethyl 2-bro...

Scheme 28: Asymmetric synthesis of methylenepyrrolidines 92 from chiral imines (RS)-14 and 2-(trimethysilylmet...

Scheme 29: Synthesis of dibenzoazaspirodecanes from cyclic N-tert-butanesulfinyl imines.

Scheme 30: Stereoselective synthesis of cyclopenta[c]proline derivatives 103 from β,γ-unsaturated α-amino acid...

Scheme 31: Stereoselective synthesis of alkaloids (−)-angustureine (107) and (−)-cuspareine (108).

Scheme 32: Stereoselective synthesis of alkaloids (−)-pelletierine (112) and (+)-coniine (117).

Scheme 33: Synthesis of piperidine alkaloids (+)-dihydropinidine (122a), (+)-isosolenopsin (122b) and (+)-isos...

Scheme 34: Stereoselective synthesis of the alkaloids(+)-sedamine (125) from chiral imine (SS)-119.

Scheme 35: Stereoselective synthesis of trans-5-hydroxy-6-substituted-2-piperidinones 127 and 129 from chiral ...

Scheme 36: Stereoselective synthesis of trans-5-hydroxy-6-substituted ethanone-2-piperidinones 132 from chiral...

Scheme 37: Stereoselective synthesis of trans-3-benzyl-5-hydroxy-6-substituted-2-piperidinones 136 from chiral...

Scheme 38: Stereoselective synthesis of trans-5-hydroxy-6-substituted 2-piperidinones 139 from chiral imine 138...

Scheme 39: Stereoselective synthesis of ʟ-hydroxypipecolic acid 145 from chiral imine 144.

Scheme 40: Synthesis of 1-substituted isoquinolones 147, 149 and 151.

Scheme 41: Stereoselective synthesis of 3-substituted dihydrobenzo[de]isoquinolinones 154.

Scheme 42: Enantioselective synthesis of alkaloids (S)-1-benzyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (...

Scheme 43: Enantioselective synthesis of alkaloids (−)-cermizine B (171) and (+)-serratezomine E (172) develop...

Scheme 44: Stereoselective synthesis of (+)-isosolepnosin (177) and (+)-solepnosin (178) from homoallylamine d...

Scheme 45: Stereoselective synthesis of tetrahydroquinoline derivatives 184, 185 and 187 from chiral imines (RS...

Scheme 46: Stereoselective synthesis of pyridobenzofuran and pyridoindole derivatives 193 from homopropargylam...

Scheme 47: Stereoselective synthesis of 2-substituted 1,2,5,6-tetrahydropyridines 196 from chiral imines (RS)-...

Scheme 48: Stereoselective synthesis of 2-substituted trans-2,6-disubstituted piperidine 199 from chiral imine...

Scheme 49: Stereoselective synthesis of cis-2,6-disubstituted piperidines 200, and alkaloid (+)-241D, from chi...

Scheme 50: Stereoselective synthesis of 6-substituted piperidines-2,5-diones 206 and 1,7-diazaspiro[4.5]decane...

Scheme 51: Stereoselective synthesis of spirocyclic oxindoles 210 from chiral imines (RS)-53.

Scheme 52: Stereoselective synthesis of azaspiro compound 213 from chiral imine 211.

Scheme 53: Stereoselective synthesis of tetrahydroisoquinoline derivatives from chiral imines (RS)-214.

Scheme 54: Stereoselective synthesis of (−)-crispine A 223 from chiral imine (RS)-214.

Scheme 55: Synthesis of (−)-harmicine (228) using tert-butanesulfinamide through haloamide cyclization.

Scheme 56: Stereoselective synthesis of tetraponerines T1–T8.

Scheme 57: Stereoselective synthesis of phenanthroindolizidines 246a and (−)-tylophorine (246b), and phenanthr...

Scheme 58: Stereoselective synthesis of indoline, tetrahydroquinoline and tetrahydrobenzazepine derivatives 253...

Scheme 59: Stereoselective synthesis of (+)-epohelmin A (258) and (+)-epohelmin B (260) from aldimine (RS)-79.

Scheme 60: Stereoselective synthesis of (−)-epiquinamide (266) from chiral aldimine (SS)-261.

Scheme 61: Synthesis synthesis of (–)-hippodamine (273) and (+)-epi-hippodamine (272) using chiral sulfinyl am...

Scheme 62: Stereoselective synthesis of (+)-grandisine D (279) and (+)-amabiline (283).

Scheme 63: Stereoselective synthesis of (−)-epiquinamide (266) and (+)-swaisonine (291) from aldimine (SS)-126....

Scheme 64: Stereoselective synthesis of (+)-C(9a)-epi-epiquinamide (294).

Scheme 65: Stereoselective synthesis of (+)-lasubine II (298) from chiral aldimine (SS)-109.

Scheme 66: Stereoselective synthesis of (−)-epimyrtine (300a) and (−)-lasubine II (ent-302) from β-amino keton...

Scheme 67: Stereoselective synthesis of (−)-tabersonine (310), (−)-vincadifformine (311), and (−)-aspidospermi...

Scheme 68: Stereoselective synthesis of (+)-epohelmin A (258) and (+)-epohelmin B (260) from aldehyde 313 and ...

Scheme 69: Total synthesis of (+)-lysergic acid (323) from N-tert-butanesulfinamide (RS)-1.
Synthetic reactions driven by electron-donor–acceptor (EDA) complexes
- Zhonglie Yang,
- Yutong Liu,
- Kun Cao,
- Xiaobin Zhang,
- Hezhong Jiang and
- Jiahong Li
Beilstein J. Org. Chem. 2021, 17, 771–799, doi:10.3762/bjoc.17.67
- spectroscopy shows that phosphono radicals could proceed throughout the reaction. A halogen bond (XB) is a noncovalent interaction formed between a halogen atom and a neutral or negatively charged Lewis base. It is a kind of weak intermolecular interaction analogous to a hydrogen bond and basically can be
- considered as a specific EDA complex [62]. In 2016, Yu and colleagues [33] employed perfluoroalkyl iodide 6 as halogen-bond donor (electron acceptor) and the organic base dibenzylamine as the halogen-bond acceptor (electron donor) to form the XB complex 8, and then a fluoroalkyl radical was given via visible
- -bond adduct. The first light-promoted three-component reaction has been realized by a halogen-bond adduct, forming perfluoroalkylated pyrimidines 26 (Scheme 8). A variety of perfluorinated chains were assembled with methylene compounds and guanidines or amidines, giving the corresponding

Graphical Abstract

Scheme 1: The electron transfer process in EDA complexes.

Scheme 2: Synthesis of benzo[b]phosphorus oxide 3 initiated by an EDA complex.

Scheme 3: Mechanism of the synthesis of quinoxaline derivative 7.

Scheme 4: Synthesis of imidazole derivative 10 initiated by an EDA complex.

Scheme 5: Synthesis of sulfamoylation product 12 initiated by an EDA complex.

Scheme 6: Mechanism of the synthesis of sulfamoylation product 12.

Scheme 7: Synthesis of indole derivative 22 initiated by an EDA complex.

Scheme 8: Synthesis of perfluoroalkylated pyrimidines 26 initiated by an EDA complex.

Scheme 9: Synthesis of phenanthridine derivative 29 initiated by an EDA complex.

Scheme 10: Synthesis of cis-tetrahydroquinoline derivative 32 initiated by an EDA complex.

Scheme 11: Mechanism of the synthesis of cis-tetrahydroquinoline derivative 32.

Scheme 12: Synthesis of phenanthridine derivative 38 initiated by an EDA complex.

Scheme 13: Synthesis of spiropyrroline derivative 40 initiated by an EDA complex.

Scheme 14: Synthesis of benzothiazole derivative 43 initiated by an EDA complex.

Scheme 15: Synthesis of perfluoroalkyl-s-triazine derivative 45 initiated by an EDA complex.

Scheme 16: Synthesis of indoline derivative 47 initiated by an EDA complex.

Scheme 17: Mechanism of the synthesis of spirocyclic indoline derivative 47.

Scheme 18: Synthesis of cyclobutane product 50 initiated by an EDA complex.

Scheme 19: Mechanism of the synthesis of spirocyclic indoline derivative 50.

Scheme 20: Synthesis of 1,3-oxazolidine compound 59 initiated by an EDA complex.

Scheme 21: Synthesis of trifluoromethylated product 61 initiated by an EDA complex.

Scheme 22: Synthesis of indole alkylation product 64 initiated by an EDA complex.

Scheme 23: Synthesis of perfluoroalkylation product 67 initiated by an EDA complex.

Scheme 24: Synthesis of hydrotrifluoromethylated product 70 initiated by an EDA complex.

Scheme 25: Synthesis of β-trifluoromethylated alkyne product 71 initiated by an EDA complex.

Scheme 26: Mechanism of the synthesis of 2-phenylthiophene derivative 74.

Scheme 27: Synthesis of allylated product 80 initiated by an EDA complex.

Scheme 28: Synthesis of trifluoromethyl-substituted alkynyl product 84 initiated by an EDA complex.

Scheme 29: Synthesis of dearomatized fluoroalkylation product 86 initiated by an EDA complex.

Scheme 30: Mechanism of the synthesis of dearomatized fluoroalkylation product 86.

Scheme 31: Synthesis of C(sp3)–H allylation product 91 initiated by an EDA complex.

Scheme 32: Synthesis of perfluoroalkylation product 93 initiated by an EDA complex.

Scheme 33: Synthesis of spirocyclic indolene derivative 95 initiated by an EDA complex.

Scheme 34: Synthesis of perfluoroalkylation product 97 initiated by an EDA complex.

Scheme 35: Synthesis of alkylated indole derivative 100 initiated by an EDA complex.

Scheme 36: Mechanism of the synthesis of alkylated indole derivative 100.

Scheme 37: Synthesis of arylated oxidized indole derivative 108 initiated by an EDA complex.

Scheme 38: Synthesis of 4-ketoaldehyde derivative 111 initiated by an EDA complex.

Scheme 39: Mechanism of the synthesis of 4-ketoaldehyde derivative 111.

Scheme 40: Synthesis of perfluoroalkylated olefin 118 initiated by an EDA complex.

Scheme 41: Synthesis of alkylation product 121 initiated by an EDA complex.

Scheme 42: Synthesis of acylation product 123 initiated by an EDA complex.

Scheme 43: Mechanism of the synthesis of acylation product 123.

Scheme 44: Synthesis of trifluoromethylation product 126 initiated by an EDA complex.

Scheme 45: Synthesis of unnatural α-amino acid 129 initiated by an EDA complex.

Scheme 46: Synthesis of thioether derivative 132 initiated by an EDA complex.

Scheme 47: Synthesis of S-aryl dithiocarbamate product 135 initiated by an EDA complex.

Scheme 48: Mechanism of the synthesis of S-aryl dithiocarbamate product 135.

Scheme 49: Synthesis of thioether product 141 initiated by an EDA complex.

Scheme 50: Mechanism of the synthesis of borate product 144.

Scheme 51: Synthesis of boronation product 148 initiated by an EDA complex.

Scheme 52: Synthesis of boration product 151 initiated by an EDA complex.

Scheme 53: Synthesis of boronic acid ester derivative 154 initiated by an EDA complex.

Scheme 54: Synthesis of β-azide product 157 initiated by an EDA complex.

Scheme 55: Decarboxylation reaction initiated by an EDA complex.

Scheme 56: Synthesis of amidated product 162 initiated by an EDA complex.

Scheme 57: Synthesis of diethyl phenylphosphonate 165 initiated by an EDA complex.

Scheme 58: Mechanism of the synthesis of diethyl phenylphosphonate derivative 165.

Scheme 59: Synthesis of (Z)-2-iodovinyl phenyl ether 168 initiated by an EDA complex.

Scheme 60: Mechanism of the synthesis of (Z)-2-iodovinyl phenyl ether derivative 168.

Scheme 61: Dehalogenation reaction initiated by an EDA complex.
Tuning the solid-state emission of liquid crystalline nitro-cyanostilbene by halogen bonding
- Subrata Nath,
- Alexander Kappelt,
- Matthias Spengler,
- Bibhisan Roy,
- Jens Voskuhl and
- Michael Giese
Beilstein J. Org. Chem. 2021, 17, 124–131, doi:10.3762/bjoc.17.13

- of iodofluorobenzene derivatives with nitro-cyanostilbenes is reported. The systematic variation of the fluorination degree and pattern indicates the relevance of the halogen bond strength for the induction of liquid crystalline properties. The modular self-assembly approach enables the efficient
- groups employed halogen bonding for the formation of liquid crystalline materials [8][9]. For instance, Palacio et al. used (E)-1-(4-(octyloxy)phenyl)-2-(2,3,5,6-tetrafluoro-4-iodophenyl)diazene as a photo-switchable halogen bond donor and investigated the light-induced phase transition of the complexes
- fluorine substitution of the aromatic halogen bond donor on the liquid crystallinity and the photo-response of halogen-bonded liquid crystals [12]. However, all reported halogen-bonded liquid crystals rely on the halogen-bond-acceptor capability of pyridyl units and so far, no study on the fluorescence

Graphical Abstract

Figure 1: Schematic representation of the modular approach towards halogen-bonded fluorescent liquid crystals....

Figure 2: Representative POM images of NO2-C10 at 94 °C (a) and NO2-C10∙∙∙F4Az at 61.5 °C (b) upon cooling fr...

Figure 3: Comparison of the mesomorphic properties of NO2-Cn, NO2-Cn∙∙∙F4St, and NO2-Cn∙∙∙F4Az (n = 8–11). Th...

Figure 4: Graphical representation of the calculated interaction energies in kJ/mol of the XB-acceptor NO2-C1...

Figure 5: Summary of the thermal behaviour of the azo complexes with decreasing fluorination degree as observ...

Figure 6: POM images of the supramolecular assemblies NO2-C10∙∙∙F3Az (a), NO2-C10∙∙∙F2Az (b) and NO2-C10∙∙∙F2...

Figure 7: Fluorescence studies of NO2-C9∙∙∙F4St. The photographs of the solid components as well as the forme...

Figure 8: Photographs of the assemblies with different alkoxy chain lengths on the NO2-Cn moiety directly aft...

Figure 9: Temperature-dependent fluorescent images of NO2-C9∙∙∙F4St showing the enhancement of emission upon ...
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- reactivity [25]. The triplet state energy of carbonyl compounds can also be transferred to halogen-containing compounds, leading to the homolytic dissociation of the carbon–halogen bond. This interaction has been studied for tert-butyl chloride, where the descendant radical is quite stabilized [26]. The

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.
Halogen-bonding-induced diverse aggregation of 4,5-diiodo-1,2,3-triazolium salts with different anions
- Xingyu Xu,
- Shiqing Huang,
- Zengyu Zhang,
- Lei Cao and
- Xiaoyu Yan
Beilstein J. Org. Chem. 2020, 16, 78–87, doi:10.3762/bjoc.16.10
- dimers, respectively, while the former shows boat conformation and the latter forms rectangle conformation. Triazolium salts form a linear polymer with polyiodide. Keywords: aggregation; 4,5-diiodo-1,2,3-triazolium salts; halogen bond; non-covalent interaction; Introduction The halogen bond (XB) is a

Graphical Abstract

Figure 1: 1,2,3-Triazole based XB donors: 1,2,3-triazole A, 1,2,3-triazolium B, 1,2,3-triazolylidene C and di...

Scheme 1: Synthesis of 4,5-diiodo-1,3-dimesityl-1,2,3-triazolium with iodide, Mes: 2,4,6-Me3C6H2.

Scheme 2: Synthesis of 4,5-diiodo-1,3-dimesityl-1,2,3-triazolium with different anion.

Figure 2: Packing structure of 2-I (top), 2-Br (middle) and 2-Cl (bottom). Hydrogen atoms have been omitted f...

Figure 3: Packing structure of 2-BF4. Hydrogen atoms have been omitted for clarity.

Figure 4: Packing structure of 2-OAc. Hydrogen atoms and solvent molecules have been omitted for clarity.

Figure 5: Packing structure of 2-TFA. Hydrogen atoms and disorder of fluorine atoms have been omitted for cla...

Figure 6: Packing structure of 2-I.1.5I2. Hydrogen atoms have been omitted for clarity.

Figure 7: Packing structure of 2-I.3.5I2. Hydrogen atoms have been omitted for clarity.

Figure 8: Packing structure of 2-BF4.0.5bpy. Hydrogen atoms and dichloromethane have been omitted for clarity....

Figure 9: 1,2,3-Triazole-based halogen model calculation: electrostatic potential surfaces mapped on total de...
A toolbox of molecular photoswitches to modulate the CXCR3 chemokine receptor with light
- Xavier Gómez-Santacana,
- Sabrina M. de Munnik,
- Tamara A. M. Mocking,
- Niels J. Hauwert,
- Shanliang Sun,
- Prashanna Vijayachandran,
- Iwan J. P. de Esch,
- Henry F. Vischer,
- Maikel Wijtmans and
- Rob Leurs
Beilstein J. Org. Chem. 2019, 15, 2509–2523, doi:10.3762/bjoc.15.244

- , exemplified by partial agonist 1c and equal full agonists 1d and 1e (VUF11418, Figure 2A). A tentative explanation for this efficacy switch includes a variation of the dihedral angle of the biaryl moiety, an increase of the electron density in the biaryl unit and/or a postulated halogen bond of the halogen
- azobenzene analogue 2a, we explored the substitution pattern of the outer aromatic ring with chlorine atoms in the ortho, meta and para-position (compounds 2b–d, respectively) to also assess the possibility of agonism provided by a halogen bond. Compound 2e, which contains a bromine atom in the ortho
- –f with E values between 30–50% at 10 µM. Interestingly, one of these compounds (4f) includes a methyl group as ortho-substituent on the outer ring and its agonist effect at PSS amounts to 32%, possibly questioning one of our hypotheses that a halogen bond is involved in inducing CXCR3 agonism

Graphical Abstract

Figure 1: Design of the CXCR3 efficacy photowitchable ligands. A,B) Schematic representation of a GPCR photoc...

Figure 2: Conformational alignment of a biaryl CXCR3 agonist with a designed azobenzene analogue. A) 2D struc...

Scheme 1: Synthetic strategies for compounds 2a–e, 3a–e, 4a–d, 4f–i and 5b,c (Y = H, Cl). Reagents and condit...

Scheme 2: Synthetic strategies for compounds 3f–h, 4e, 6b, and 6d (Y = H, F, Cl, Br). Reagents and conditions...

Figure 3: Comparison of compounds belonging to the subseries 3 or 4 with a halogen substitution on the ortho-...

Scheme 3: Synthetic strategy for compound 6e. Reagents and conditions: (a) i) K2CO3 (2.0 equiv), DMF, µW, 65 ...

Scheme 4: Synthetic strategies for compounds 6f–h (Y = OMe, OiPr, SMe). Reagents and conditions: (a) NaOMe or...

Figure 4: Properties of subseries 3e, 4d, 6b and 6d-h. (A) UV–vis absorption spectra of (top) trans-isomers o...
1,2,3-Triazolium macrocycles in supramolecular chemistry
- Mastaneh Safarnejad Shad,
- Pulikkal Veettil Santhini and
- Wim Dehaen
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- -triazole units to more electrophilic 1,2,3-triazolium units by influencing both hydrogen bonding-like and anion–π interactions. Moreover, halogen bond (XB) and chalcogen bond (ChB) interactions (see Figure 1) also been applied for the selective detection of anions by exchanging C5–H protons with halogens

Graphical Abstract

Figure 1: Hydrogen, halogen or chalcogen bonding to anions within a bistriazolium macrocycle.

Figure 2: Main synthetic strategies towards macrocyclic triazoliums.

Figure 3: Chemical structure of compound 1 (1a, 1b and 1c) and 2.

Figure 4: Chemical structure of compound 3 and 4.

Figure 5: Chemical structure of compound 5.

Figure 6: Chemical structure of compound 6.

Figure 7: Chemical structure of compound 7.

Figure 8: Chemical structure of compound 8.

Figure 9: Chemical structures of compound 9.

Figure 10: Chemical structures of compound 10, 11 and 12.

Figure 11: Chemical structure of compound 13.

Figure 12: Chemical structure of compound 15 including the sigma-connected TCNQ dimer.

Figure 13: Chemical structure of compound 16 for the kinetic resolution of epoxides.

Figure 14: Chemical structure of compound 17a (bisnaphtho crown ether shown).

Figure 15: Jump rope in molecular double-lasso compounds 18.

Figure 16: Chemical structure of compound 19 and acid–base triggered motions.
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
- Esther Nieland,
- Oliver Weingart and
- Bernd M. Schmidt
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197
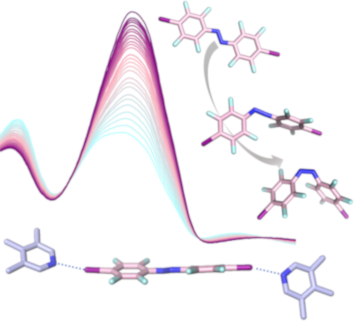
- azobenzenes with different halogen bonding donor properties are discussed in relation to their changing photophysical properties, rationalized by DFT calculations. Keywords: azobenzene; DFT calculations; fluorine chemistry; halogen bonding; photochemistry; Introduction The halogen bond is an attractive
- noncovalent interaction between a polarized halogen atom (the halogen bond donor) and a Lewis base (the halogen bond acceptor) [1][2]. A prominent example regarding the origin of halogen bonding can be found in inorganic solid-state chemistry. The structurally diverse group of polyiodides, with its rich
- structural chemistry is governed by halogen bonding, where I− and I3− are considered the nucleophilic (halogen bond acceptor) and I2 the electrophilic (halogen bond donor) subcomponent [3][4][5][6][7]. Neutral halogen bonds on the other hand can be generally described by R–X···Y, where R–X is the halogen

Graphical Abstract

Scheme 1: a) Azobenzenes A1–3 employed in this study. b) U-shaped anthracene halogen bond acceptor bearing tw...

Figure 1: Electrostatic potential map at different isodensity values (B3LYP/ def2/TZVP/DGZVP optimized geomet...

Figure 2: Top: Vertical electronic absorption spectra of a) A2 and b) A3, calculated using TD-B3LYP/def2-TZVP...

Figure 3: a) Space-filling model of U1···A2. The kinked alignment of both the lutidine units of U1 and the az...
Halogen bonding and host–guest chemistry between N-alkylammonium resorcinarene halides, diiodoperfluorobutane and neutral guests
- Fangfang Pan,
- Mohadeseh Dashti,
- Michael R. Reynolds,
- Kari Rissanen,
- John F. Trant and
- Ngong Kodiah Beyeh
Beilstein J. Org. Chem. 2019, 15, 947–954, doi:10.3762/bjoc.15.91

- chloride (2), and 1,4-diiodooctafluorobutane and accompanying small solvent guests (methanol, acetonitrile and water) are presented. The guests’ inclusion affects the geometry of the cavity of the receptors 1 and 2, while the divalent halogen bond donor 1,4-diiodooctafluorobutane determines the overall
- nature of the halogen bond assembly. The crystal lattice of 1 contains two structurally different dimeric assemblies A and B, formally resulting in the mixture of a capsular dimer and a dimeric pseudo-capsule. 1H and 19F NMR analyses supports the existence of these halogen-bonded complexes and enhanced
- halogen bond acceptors [29][30][31][32][33][34]. We have previously shown that N-alkylammonium resorcinarene bromides (NARBrs) can form various halogen-bonded assemblies with the classical organic halogen bond donor 1,4-diiodooctafluorobutane (DIOFB) depending on the solvent, the presence of potential

Graphical Abstract

Figure 1: Tetravalent XB acceptor, Hex-NARBr 1, Cy-NARCl 2, divalent XB donor DIOFB, and guests MeCN, MeOH an...

Figure 2: The previously reported halogen-bonded complexes CHCl3@1&DIOFB (a), and MeOH@1&DIOFB (b). (c)The fi...

Figure 3: The two dimerization modes in the MeOH-MeCN@1&DIOFB complex. In both modes, the cavities are shown ...

Figure 4: (a) The halogen bonding (blue broken lines), hydrogen bonding (red broken lines) and the host–guest...

Figure 5: 19F NMR in CDCl3 at 298 K of: a) DIOFB (10 mM); b) 1:2 mixture of DIOFB and 1; 1:2:1 mixture of DIO...

Figure 6: 1H NMR in CDCl3 at 298 K of: a) 1 (10 mM), b) 1:2 mixture of 1 and DIOFB, c) 1:2:1 mixture of 1, DI...
Electrochemically modified Corey–Fuchs reaction for the synthesis of arylalkynes. The case of 2-(2,2-dibromovinyl)naphthalene
- Fabiana Pandolfi,
- Isabella Chiarotto and
- Marta Feroci
Beilstein J. Org. Chem. 2018, 14, 891–899, doi:10.3762/bjoc.14.76
- reagents and frequently these reactions are carried out at room temperature and at atmospheric pressure, etc. [16][17][18][19]. The electrochemical behavior of halogenated compounds has been extensively investigated [20][21][22]. The cleavage of the C–halogen bond, yielding (via a radical intermediate) the
- corresponding carbanion and halogen anion, can be achieved by a bielectronic cathodic process (Scheme 2). The electrolysis is carried out at a suitable controlled potential, i.e., at a potential that is negative enough to achieve the selective fission of the envisaged C–halogen bond [23]. Therefore, the
- = 25 °C; solvent left: DMF/Et4NBF4 0.1 mol dm−3; right: ACN/Et4NBF4 0.1 mol dm−3. Variation of the amounts of 1a, 2a, and 3a with the number of Faradays of 1a. The Corey–Fuchs reaction. Electrochemical reduction of a carbon–halogen bond. Electrochemical synthesis of vinyl bromides [25]. Scope of this

Graphical Abstract

Scheme 1: The Corey–Fuchs reaction.

Scheme 2: Electrochemical reduction of a carbon–halogen bond.

Scheme 3: Electrochemical synthesis of vinyl bromides [25].

Scheme 4: Scope of this work.

Figure 1: Voltammetric curves of 1a 0.020 mol dm−3; Pt, glassy carbon (GC) or Ag cathode. ν = 0.2 V s−1, T = ...

Scheme 5: Possible products from the electrolysis of 2-(2,2-dibromovinyl)naphthalene (1a).

Figure 2: Variation of the amounts of 1a, 2a, and 3a with the number of Faradays of 1a.

Scheme 6: Mechanistic hypothesis for the synthesis of alkyne 2a and bromoalkyne 3a from 2-(2,2-dibromovinyl)n...

Scheme 7: Possible reaction using NaClO4 as supporting electrolyte.

Scheme 8: Electrochemical synthesis of 9-ethyl-3-ethynyl-9H-carbazole (2b).

Scheme 9: Electrochemical synthesis of 1-ethynyl-4-methoxybenzene (2c).
Progress in copper-catalyzed trifluoromethylation
- Guan-bao Li,
- Chao Zhang,
- Chun Song and
- Yu-dao Ma
Beilstein J. Org. Chem. 2018, 14, 155–181, doi:10.3762/bjoc.14.11
- allylic halides were also subjected to these conditions. It is notable that the trifluoromethyl group was introduced at the α position of the carbon–halogen bond with complete regioselectivity. Later, they extended these conditions to propargylic halides [27] and succeeded in synthesizing the

Graphical Abstract

Figure 1: Selected examples of pharmaceutical and agrochemical compounds containing the trifluoromethyl group....

Scheme 1: Introduction of a diamine into copper-catalyzed trifluoromethylation of aryl iodides.

Scheme 2: Addition of a Lewis acid into copper-catalyzed trifluoromethylation of aryl iodides and the propose...

Scheme 3: Trifluoromethylation of heteroaromatic compounds using S-(trifluoromethyl)diphenylsulfonium salts a...

Scheme 4: The preparation of a new trifluoromethylation reagent and its application in trifluoromethylation o...

Scheme 5: Trifluoromethylation of aryl iodides using CF3CO2Na as a trifluoromethyl source.

Scheme 6: Trifluoromethylation of aryl iodides using MTFA as a trifluoromethyl source.

Scheme 7: Trifluoromethylation of aryl iodides using CF3CO2K as a trifluoromethyl source.

Scheme 8: Trifluoromethylation of aryl iodides and heteroaryl bromides using [Cu(phen)(O2CCF3)] as a trifluor...

Scheme 9: Trifluoromethylation of aryl iodides with DFPB and the proposed mechanism.

Scheme 10: Trifluoromethylation of aryl iodides using TCDA as a trifluoromethyl source. Reaction conditions: [...

Scheme 11: The mechanism of trifluoromethylation using Cu(II)(O2CCF2SO2F)2 as a trifluoromethyl source.

Scheme 12: Trifluoromethylation of benzyl bromide reported by Shibata’s group.

Scheme 13: Trifluoromethylation of allylic halides and propargylic halides reported by the group of Nishibayas...

Scheme 14: Trifluoromethylation of propargylic halides reported by the group of Nishibayashi.

Scheme 15: Trifluoromethylation of alkyl halides reported by Nishibayashi’s group.

Scheme 16: Trifluoromethylation of pinacol esters reported by the group of Gooßen.

Scheme 17: Trifluoromethylation of primary and secondary alkylboronic acids reported by the group of Fu.

Scheme 18: Trifluoromethylation of boronic acid derivatives reported by the group of Liu.

Scheme 19: Trifluoromethylation of organotrifluoroborates reported by the group of Huang.

Scheme 20: Trifluoromethylation of aryl- and vinylboronic acids reported by the group of Shibata.

Scheme 21: Trifluoromethylation of arylboronic acids via the merger of photoredox and Cu catalysis.

Scheme 22: Trifluoromethylation of arylboronic acids reported by Sanford’s group. Isolated yield. aYields dete...

Scheme 23: Trifluoromethylation of arylboronic acids and vinylboronic acids reported by the group of Beller. Y...

Scheme 24: Copper-mediated Sandmeyer type trifluoromethylation using Umemoto’s reagent as a trifluoromethylati...

Scheme 25: Copper-mediated Sandmeyer type trifluoromethylation using TMSCF3 as a trifluoromethylation reagent ...

Scheme 26: One-pot Sandmeyer trifluoromethylation reported by the group of Gooßen.

Scheme 27: Copper-catalyzed trifluoromethylation of arenediazonium salts in aqueous media.

Scheme 28: Copper-mediated Sandmeyer trifluoromethylation using Langlois’ reagent as a trifluoromethyl source ...

Scheme 29: Trifluoromethylation of terminal alkenes reported by the group of Liu.

Scheme 30: Trifluoromethylation of terminal alkenes reported by the group of Wang.

Scheme 31: Trifluoromethylation of tetrahydroisoquinoline derivatives reported by Li and the proposed mechanis...

Scheme 32: Trifluoromethylation of phenol derivatives reported by the group of Hamashima.

Scheme 33: Trifluoromethylation of hydrazones reported by the group of Baudoin and the proposed mechanism.

Scheme 34: Trifluoromethylation of benzamides reported by the group of Tan.

Scheme 35: Trifluoromethylation of heteroarenes and electron-deficient arenes reported by the group of Qing an...

Scheme 36: Trifluoromethylation of N-aryl acrylamides using CF3SO2Na as a trifluoromethyl source.

Scheme 37: Trifluoromethylation of aryl(heteroaryl)enol acetates using CF3SO2Na as the source of CF3 and the p...

Scheme 38: Trifluoromethylation of imidazoheterocycles using CF3SO2Na as a trifluoromethyl source and the prop...

Scheme 39: Copper-mediated trifluoromethylation of terminal alkynes using TMSCF3 as a trifluoromethyl source a...

Scheme 40: Improved copper-mediated trifluoromethylation of terminal alkynes reported by the group of Qing.

Scheme 41: Copper-catalyzed trifluoromethylation of terminal alkynes reported by the group of Qing.

Scheme 42: Copper-catalyzed trifluoromethylation of terminal alkynes using Togni’s reagent and the proposed me...

Scheme 43: Copper-catalyzed trifluoromethylation of terminal alkynes using Umemoto’s reagent reported by the g...

Scheme 44: Copper-catalyzed trifluoromethylation of 3-arylprop-1-ynes reported by Xiao and Lin and the propose...
Stereochemical outcomes of C–F activation reactions of benzyl fluoride
- Neil S. Keddie,
- Pier Alexandre Champagne,
- Justine Desroches,
- Jean-François Paquin and
- David O'Hagan
Beilstein J. Org. Chem. 2018, 14, 106–113, doi:10.3762/bjoc.14.6
- carbon–halogen bond known [1]. Its low reactivity, in comparison to other C–X bonds, means that it is inert to all but the most harsh reaction conditions, and fluorine can generally be carried through multistep syntheses without concern over side reactions (the exception being SNAr reactions). In recent

Graphical Abstract

Figure 1: C–F activation of benzylic fluorides to generate benzylamine or diarylmethane products.

Figure 2: 7-[2H1]-(R)-Benzyl fluoride ((R)-1).

Scheme 1: Synthesis of enantioenriched 7-[2H1]-(R)-benzyl fluoride ((R)-1) from benzaldehyde (2).

Figure 3: Partial 2H{1H} NMR (107.5 MHz) with PBLG in CHCl3 (13% w/w). (A) racemic sample of 6 (from Table 1, entry ...

Scheme 2: Synthesis of enantioenriched (S)-diarylmethane 10 from diaryl ketone 11 and confirmation of configu...

Figure 4: Possible reactive intermediates for C–F activation of benzyl fluoride 1 with strong hydrogen bond d...
Conformational preferences of α-fluoroketones may influence their reactivity
- Graham Pattison
Beilstein J. Org. Chem. 2017, 13, 2915–2921, doi:10.3762/bjoc.13.284
- -haloacetophenone, calculating the energy of each compound as the carbon–halogen bond is rotated through 10° increments in both the gas phase and in ethanol as reaction solvent (Figure 1) [10]. The fluorinated acetophenone showed significant differences in conformational energy to the chlorinated and brominated

Graphical Abstract

Scheme 1: Relative reactivity of α-fluoroacetophenone to α-chloroacetophenone and α-bromoacetophenone.

Scheme 2: Competitive reduction of haloacetophenones and acetophenone.

Figure 1: Conformational energy profiles of halogenated acetophenones (a) in gas phase; (b) in EtOH; (c) over...

Figure 2: Optimised gas phase geometries of (a) α-fluoroacetophenone and (b) α-chloroacetophenone emphasising...

Figure 3: Most stable conformations of (a) α-fluoroacetophenone and (b) α-chloroacetophenone in ethanol.

Figure 4: Expected reactive conformation of halo-acetophenones.

Figure 5: Orbital interactions in gauche- and cis-conformations of haloacetophenones.

Figure 6: Variation of dipole moment with angle for haloacetophenones.

Figure 7: Highest energy conformation of fluoroacetophenone, emphasizing the closeness of approach of fluorin...

Scheme 3: Competitive reduction of fluoroacetone and chloroacetone.

Figure 8: Conformational energy profiles of halogenated acetones in gas phase and in MeOH.

Figure 9: Overlay of conformational energy profiles of fluoroacetone and fluoroacetophenone.
CF3SO2X (X = Na, Cl) as reagents for trifluoromethylation, trifluoromethylsulfenyl-, -sulfinyl- and -sulfonylation and chlorination. Part 2: Use of CF3SO2Cl
- Hélène Chachignon,
- Hélène Guyon and
- Dominique Cahard
Beilstein J. Org. Chem. 2017, 13, 2800–2818, doi:10.3762/bjoc.13.273
- of an halogen bond between the positive electrostatic potential on the outer side of the chlorine atom and the lone pair of the phosphorus atom of the phosphine. This phenomenon indeed triggered the cleavage of the S–Cl bond, producing chlorophosphonium sulfinate 40, which was then readily converted

Graphical Abstract

Scheme 1: Trifluoromethylation of silyl enol ethers.

Scheme 2: Continuous flow trifluoromethylation of ketones under photoredox catalysis.

Scheme 3: Trifluoromethylation of enol acetates.

Scheme 4: Photoredox-catalysed tandem trifluoromethylation/cyclisation of N-arylacrylamides: a route to trifl...

Scheme 5: Tandem trifluoromethylation/cyclisation of N-arylacrylamides using BiOBr nanosheets catalysis.

Scheme 6: Photoredox-catalysed trifluoromethylation/desulfonylation/cyclisation of N-tosyl acrylamides (bpy: ...

Scheme 7: Photoredox-catalysed trifluoromethylation/aryl migration/desulfonylation of N-aryl-N-tosylacrylamid...

Scheme 8: Proposed mechanism for the trifluoromethylation/aryl migration/desulfonylation (/cyclisation) of N-...

Scheme 9: Photoredox-catalysed trifluoromethylation/cyclisation of N-methacryloyl-N-methylbenzamide derivativ...

Scheme 10: Photoredox-catalysed trifluoromethylation/cyclisation of N-methylacryloyl-N-methylbenzamide derivat...

Scheme 11: Photoredox-catalysed trifluoromethylation/dearomatising spirocyclisation of a N-benzylacrylamide de...

Scheme 12: Photoredox-catalysed trifluoromethylation/cyclisation of an unactivated alkene.

Scheme 13: Asymmetric radical aminotrifluoromethylation of N-alkenylurea derivatives using a dual CuBr/chiral ...

Scheme 14: Aminotrifluoromethylation of an N-alkenylurea derivative using a dual CuBr/phosphoric acid catalyti...

Scheme 15: 1,2-Formyl- and 1,2-cyanotrifluoromethylation of alkenes under photoredox catalysis.

Scheme 16: First simultaneous introduction of the CF3 moiety and a Cl atom onto alkenes.

Scheme 17: Chlorotrifluoromethylaltion of terminal, 1,1- and 1,2-substituted alkenes.

Scheme 18: Chorotrifluoromethylation of electron-deficient alkenes (DCE = dichloroethane).

Scheme 19: Cascade trifluoromethylation/cyclisation/chlorination of N-allyl-N-(benzyloxy)methacrylamide.

Scheme 20: Cascade trifluoromethylation/cyclisation (/chlorination) of diethyl 2-allyl-2-(3-methylbut-2-en-1-y...

Scheme 21: Trifluoromethylchlorosulfonylation of allylbenzene derivatives and aliphatic alkenes.

Scheme 22: Access to β-hydroxysulfones from CF3-containing sulfonyl chlorides through a photocatalytic sequenc...

Scheme 23: Cascade trifluoromethylchlorosulfonylation/cyclisation reaction of alkenols: a route to trifluorome...

Scheme 24: First direct C–H trifluoromethylation of arenes and proposed mechanism.

Scheme 25: Direct C–H trifluoromethylation of five- and six-membered (hetero)arenes under photoredox catalysis....

Scheme 26: Alternative pathway for the C–H trifluoromethylation of (hetero)arenes under photoredox catalysis.

Scheme 27: Direct C–H trifluoromethylation of five- and six-membered ring (hetero)arenes using heterogeneous c...

Scheme 28: Trifluoromethylation of terminal olefins.

Scheme 29: Trifluoromethylation of enamides.

Scheme 30: (E)-Selective trifluoromethylation of β-nitroalkenes under photoredox catalysis.

Scheme 31: Photoredox-catalysed trifluoromethylation/cyclisation of an o-azidoarylalkynes.

Scheme 32: Regio- and stereoselective chlorotrifluoromethylation of alkynes.

Scheme 33: PMe3-mediated trifluoromethylsulfenylation by in situ generation of CF3SCl.

Scheme 34: (EtO)2P(O)H-mediated trifluoromethylsulfenylation of (hetero)arenes and thiols.

Scheme 35: PPh3/NaI-mediated trifluoromethylsulfenylation of indole derivatives.

Scheme 36: PPh3/n-Bu4NI mediated trifluoromethylsulfenylation of thiophenol derivatives.

Scheme 37: PPh3/Et3N mediated trifluoromethylsulfinylation of benzylamine.

Scheme 38: PCy3-mediated trifluoromethylsulfinylation of azaarenes, amines and phenols.

Scheme 39: Mono- and dichlorination of carbon acids.

Scheme 40: Monochlorination of (N-aryl-N-hydroxy)acylacetamides.

Scheme 41: Examples of the synthesis of heterocycles fused with β-lactams through a chlorination/cyclisation p...

Scheme 42: Enantioselective chlorination of β-ketoesters and oxindoles.

Scheme 43: Enantioselective chlorination of 3-acyloxazolidin-2-one derivatives (NMM = N-methylmorpholine).
Pyrene–nucleobase conjugates: synthesis, oligonucleotide binding and confocal bioimaging studies
- Artur Jabłoński,
- Yannic Fritz,
- Hans-Achim Wagenknecht,
- Rafał Czerwieniec,
- Tytus Bernaś,
- Damian Trzybiński,
- Krzysztof Woźniak and
- Konrad Kowalski
Beilstein J. Org. Chem. 2017, 13, 2521–2534, doi:10.3762/bjoc.13.249

- observed in the molecular structures of the metallocene–nucleobase derivatives [24][29]. In addition, each molecule of 2 in the dimer is further involved in a Cl···O halogen bond with an adjacent molecule of chloroform (Figure 3). Further details of intermolecular interactions present in the crystal

Graphical Abstract

Figure 1: Examples of pyrene derivatives with relevance to nucleic acid chemistry and structures of pyrenyl–n...

Scheme 1: Synthesis of pyrene–nucleobase conjugates 2–5.

Figure 2: ORTEP diagram of 2 at 50% probability level. The hydrogen and halogen bonds are represented by dash...

Figure 3: Intermolecular hydrogen bonding (N22–H22···O27 distance = 2.882(2) Å) and halogen bonding (C30–Cl31...

Figure 4: UV–vis absorption and fluorescence spectra of pyrene–adenines 5 (a) and 3 (b) in diluted (c ≈ 10−5 ...

Figure 5: Absorption changes during titration of 2 and 4 (λ = 344 nm) in the presence of (dA)10, and 3 and 5 ...

Figure 6: Cellular distribution of 4 in living HeLa cells. (A) Fluorescence of 4 (green). (B) Fluorescence of...

Figure 7: Cellular distribution of 5 in living HeLa cells. (A) Fluorescence of 5 (green). Arrows are marking ...
Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1,2,3-triazole
- Martina Tireli,
- Silvija Maračić,
- Stipe Lukin,
- Marina Juribašić Kulcsár,
- Dijana Žilić,
- Mario Cetina,
- Ivan Halasz,
- Silvana Raić-Malić and
- Krunoslav Užarević
Beilstein J. Org. Chem. 2017, 13, 2352–2363, doi:10.3762/bjoc.13.232
- bands at 1099 (Cl), 1077 (Br) and 1064 (I) cm−1 which are assigned to vibration of the phenyl ring that contains the carbon–halogen bond. Characteristic C≡C alkyne band at 2133 cm−1 along with the band at 1258 cm−1 of the triazole products are appropriate for monitoring of the reaction progress. In situ

Graphical Abstract

Scheme 1: Synthetic procedures for preparation of p-halogen-substituted and non-substituted phenyl-1,2,3-tria...

Figure 1: Experimental Raman spectra of the alkyne 4 and triazole products 5–8. Bands attributed to the vibra...

Figure 2: In situ Raman monitoring of a) mechanochemical formation of triazole 5 using copper(II) acetate mon...

Figure 3: a) In situ Raman monitoring for mechanochemical synthesis of 5 using brass balls and PMMA reaction ...

Figure 4: ESR spectra of samples obtained after milling by methods 2a (black), 2b (red) and 2c (blue). The in...

Figure 5: X-ray structure of the triazole compounds. (a) Molecular structure of 5, with the atom-numbering sc...
Detection of therapeutic radiation in three-dimensions
- John A. Adamovics
Beilstein J. Org. Chem. 2017, 13, 1325–1331, doi:10.3762/bjoc.13.129
- consistent with the bond energy of the carbon–halogen bond. The observed sensitivity was in the order R3C–I > R3C–Br > R3C–Cl [42][43][44]. Due to the high electron density of radical initiators containing iodine even at relatively low concentrations (100 mM) result in dosimeters that are not tissue

Graphical Abstract

Scheme 1: Ionizing radiation reactions in the Fricke dosimeter.

Figure 1: Structure of xylenol orange.

Scheme 2: Sulfuric acid/urea promoted synthesis of LMG.

Figure 2: Aliphatic diisocyantes HMDI, HDI, IPDI.

Figure 3: Absorption spectrum of irradiated leucomalachite green.

Figure 4: 3D dosimeters fabricated in our lab for a variety of radiation therapies. Top left a head dosimeter...

Figure 5: OCT scanner used in our lab to create 3D images.






